Licensed Global Pharmacopeia Access with side-by-side comparison and full official texts from USP–NF, British Pharmacopoeia (BP), European Pharmacopoeia (Ph. Eur.), Japanese Pharmacopoeia (JP), Indian Pharmacopoeia (IP), Chinese Pharmacopoeia (ChP), and others.
Search and navigate monographs across multiple pharmacopeias (USP–NF, BP, Ph. Eur., JP, IP, WHO, ChP) from a single, elegant interface.
Side-by-side comparisons of identification tests, assays, impurity limits, dissolution, and acceptance criteria—highlighting differences.
Track editions and revisions; view redlined changes between releases to accelerate method updates and change-control.
Export curated summaries into Word/PDF with citations for QA/QC, Regulatory Affairs, and internal audits.
Role-based permissions, SSO, and audit logs to meet compliance needs across teams and regions.
Each insight links back to its authoritative section so reviewers can verify quickly.
Arabic/English UI, right-to-left support, and localized terminology mappings for regional teams.
Connect to LIMS, ELN, or ERP to pull product lists, push reports, and synchronize approvals.
Private cloud or on-premise deployments with regional hosting and encryption at rest/in transit.
Organizations subscribe to MonographHub to gain secure, licensed access to supported pharmacopeias.
Browse monographs, compare sections across multiple pharmacopeias, and extract key differences instantly.
Keep citations to chapters/sections, then export audit-ready Word/PDF with change-logs for QA/QC and regulatory use.
Browse real conversations with the MonographHub AI. Use the tabs to switch between screenshots.
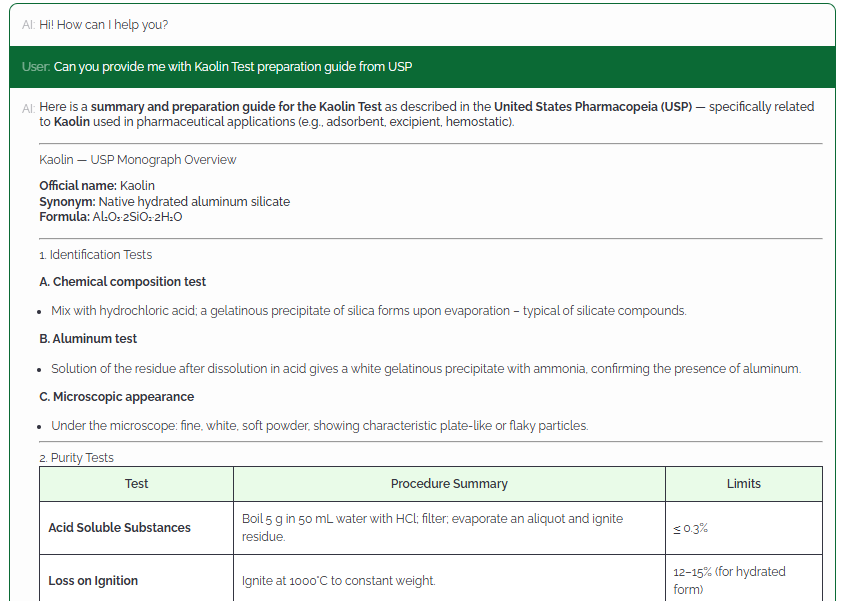
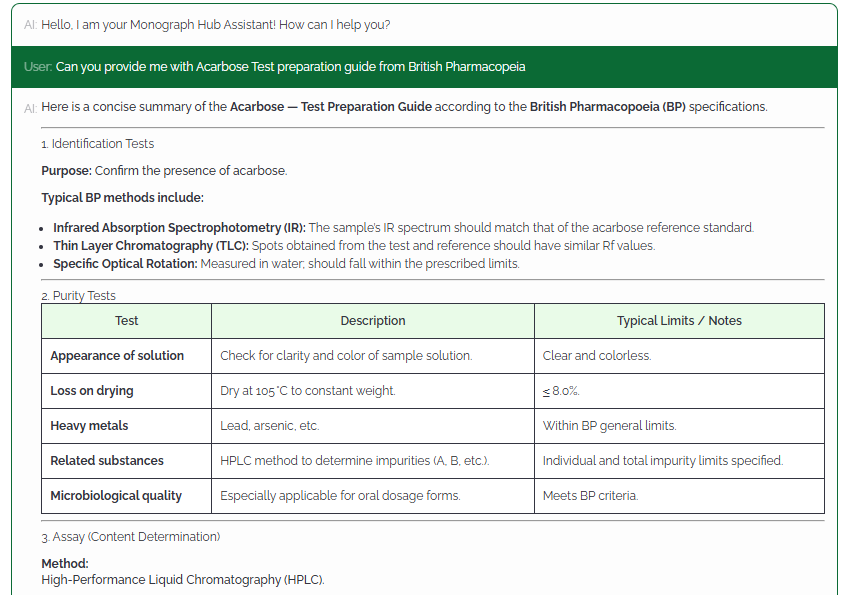
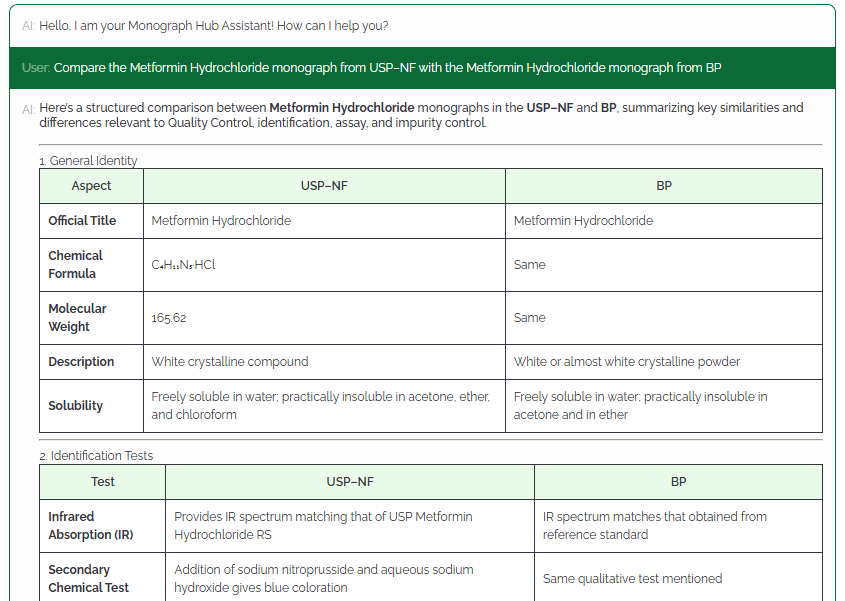
MonographHub unifies licensed access to global pharmacopeias and layers AI-assisted analysis to help QA/QC, Regulatory, and R&D teams compare, verify, and export official monograph data with complete traceability.
Make compliant, up-to-date pharmacopeial knowledge accessible and comparable in seconds—accelerating reviews and reducing risk.
Side-by-side comparisons, change tracking, citation-preserving exports, and enterprise controls—built for real-world audits.
Pharmaceutical manufacturers, CROs, and academic labs that need verified standards across USP, BP, Ph. Eur., JP, IP, ChP, WHO, and more.
Subscribe today and gain full access to USP, BP, Ph. Eur., JP, IP, ChP, WHO and more — all within a unified, AI-ready platform.